Answer:
V₁ = 1.75 m³
Step-by-step explanation:
Assuming the gas to be an ideal gas. At constant temperature, the relationship between the volume and temperature of an ideal gas is given by Boyle's Law as follows:
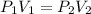
where,
P₁ = Initial Pressure of the Gas = 4 KPa
V₁ = Initial Volume of the Gas = ?
P₂ = Final Pressure of the Gas = 2 KPa
V₂ = Final Volume of the Gas = 3.5 m³
Therefore,
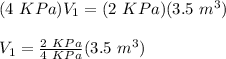
V₁ = 1.75 m³