Answer: H
H- C- H
H
Step-by-step explanation:
Lewis structures are the structures which represent the valence electrons of the element around the chemical symbol of the element.
Carbon is an element which belong to group 14. Atomic number of carbon is 6 that means it contains 6 electrons, Thus the electronic configuration is represented as:
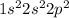 . Thus the valence electrons are 4.
. Thus the valence electrons are 4.
Hydrogen is an element which belong to group 1. Atomic number of hydrogen is 1 that means it contains 1 electron, Thus the electronic configuration is represented as:
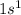 . Thus the valence electron are 1.
. Thus the valence electron are 1.
The four valence electrons of carbon are shared with one valence electron of hydrogen each.