Answer:
A. 1.02 moles .
Step-by-step explanation:
Hello!
In this case, given the ideal gas equation, as we need to solve for moles, we divide both sides by RT to get:
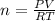
Thus, by plugging in the pressure and temperature at STP (1.00 atm and 273.15 K respectively) we obtain:
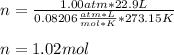
Therefore, the correct answer is A. 1.02 moles
Best regards!