Answer:
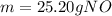
Step-by-step explanation:
Hello!
In this case, since the STP conditions are known as 273.15 K of temperature and 1.00 atm of pressure, we can use the ideal gas equation to compute the moles of NO gas first:

Next, since the molar mass of NO is 30.01 g/mol the resulting mass in grams is:
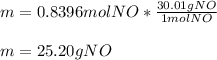
Which has four significant figures.
Best regards!