Answer:
Mass, m = 141.96 kg
Step-by-step explanation:
Given that,
Moles = 5.07
Molar mass of N₂ = 28 g/mol
We need to find the mass of 5.07 mol N₂.
We know that,
No of moles is equal to mass divided by molar mass i.e.
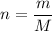
Where
m is mass of 5.07 mol of N₂
So,
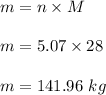
So, the required mass of 5.07 mol N₂ is 141.96 kg.