Answer:
21.5 g.
Step-by-step explanation:
Hello!
In this case, since the reaction between the given compounds is:
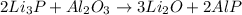
We can see that according to the law of conservation of mass, which states that matter is neither created nor destroyed during a chemical reaction, the total mass of products equals the total mass of reactants based on the stoichiometric proportions; in such a way, we first need to compute the reacted moles of Li3P as shown below:
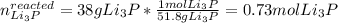
Now, the moles of Li3P consumed by 15 g of Al2O3:
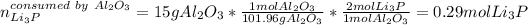
Thus, we infer that just 0.29 moles of 0.73 react to form products; which means that the mass of formed products is:
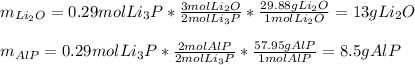
Therefore, the total mass of products is:
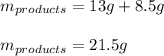
Which is not the same to the reactants (53 g) because there is an excess of Li₃P.
Best Regards!