Answer: The correct answer is: " 0.54 g " .
__________________________________________
Step-by-step explanation:
Note that "hydrogen gas" is:
H₂ (g) ; that is: a "diatomic element" (diatomic gas) ;
_________________________________________
The molecular weight of "H" is: 1.00794 g ;
(From the Periodic Table of Elements).
So, the molecular weight of: H₂ (g) is:
" 1.00794 g * 2 = 2.01588 g ; {use calculator) ;
_________________________________________
Note the conversion for a gas at STP:
______
1 mol of a gas = 22.4 L gas;
___
i.e. " 1 mol / 22.4 L " ;
____
So: " 5.7 L H₂ (g)
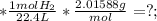
The "L" ("literes" cancel out to "1" ; since "L/L = 1 ;
The "mol" (moles) cancel out to "1" ; since "mol/mol = 1 ;
____
and we are left with:
____
[5.7 * 2.104588 g ] / 22.4 = ? g ;
______________________
→ [ 11.9961516 g ] / 22.4 =
0.53554248214 g ;l
_____________________________
We round this value to: " 0.54 g " ;
→ since "5.7 L " has 2 (two) significant figures;
22.4 is an exact number conversion;
and "5.7 L" has fewer significant figures than:
" 2.104588 " ; or: " 1.00794 " .
→ as such: We round to "2 (two) significant figures."
______________________________
Hope this is helpful. Wishing you the best in your academic endeavors!
_______________________________