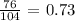Answer:
Step-by-step explanation:
Here, we start by calculating the formula mass of the compound
We can get this by using the atomic masses of the elements
The atomic mass of silicon is 28 amu
The atomic mass of fluorine is 19 amu
The formula mass of the given compound is thus:
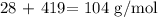
Now, we divide the individual total mass by element by the formula mass
For Silicon, we have:
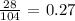
For Fluorine, we have: