Answer:
a) F = 1.70 10⁻⁹N, F = 1.47 10⁻⁸ N,
b) * the electronegative repulsion, from the repulsion by quantum effects
Step-by-step explanation:
a) The atraicione force comes from the electric force given by Coulomb's law,
F =
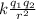
divalent atoms
In this case q = 2q₀ where qo is the charge of the electron -1,6 10⁻¹⁹ C and the separation is given
F = k q² / r²
F =
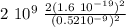
F = 1.70 10⁻⁹N
monovalent atoms
in this case the load is q = q₀
F = 2 \ 10^9 \ \frac{ (1.6 \ 10^{-19} )^2}{ (0.125 10^{-9} )^2 }
F = 1.47 10⁻⁸ N
b) repulsive forces come from various sources
* the electronegative repulsion of positive nuclei
* the electrostatic repulsion of the electrons when it comes to bringing the electron clouds closer together
* from the repulsion of electron clouds, by quantum effects