Answer
Explanations:
The formula for calculating the percent impurity of oxygen is expressed as:
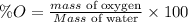
Given the following parameter
Mass of water = 36.8 grams
Determine the moles of water
According to the ideal gas equation, PV = nRT

Determine the mass of oxygen
Mass of O2 = moles * molar mass
Mass of O2 = 2.857 * 16
Mass of O2 =