Answer : The volume of concentrated hydrochloric acid is, 0.25 liters
Explanation :
Using neutralization law,

where,
 = molarity of concentrated hydrochloric acid = 12 M = 12 mole/L
= molarity of concentrated hydrochloric acid = 12 M = 12 mole/L
 = molarity of hydrochloric acid = 6 M = 6 mole/L
= molarity of hydrochloric acid = 6 M = 6 mole/L
 = volume of concentrated hydrochloric acid = ?
= volume of concentrated hydrochloric acid = ?
 = volume of hydrochloric acid = 500 ml = 0.5 L
= volume of hydrochloric acid = 500 ml = 0.5 L
Now put all the given values in the above formula, we get the volume of concentrated hydrochloric acid.
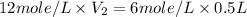
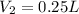
Therefore, the volume of concentrated hydrochloric acid is, 0.25 liters