Answer:
x = 1382.9 km
Step-by-step explanation:
The speed of the wave is constant, so we can use the uniform motion relationships
p wave
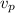 = x / t₁
= x / t₁
t₁ = x /v_p
S wave
v_s = x / t₂
t₂ = x / v_s
indicate that the time difference between the two waves is
t₂ - t₁ = 3.25 min (60 s / 1 min)
t₂ -t₁ = 195 s
let's substitute
 = 195
= 195
x (
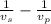 = 195
= 195
let's calculate
x
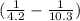 = 195
= 195
x (0.1410) = 195
x = 195 /0.141
x = 1382.9 km