Step-by-step explanation:
The net ionic equation is found by determining the ionic state of each particle involved in the reaction.
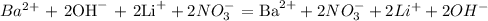
The chemical equation above only includes particles that are invloved in the reactant and product side. Therefore, the net ionic equation is found by removing particles that are on the reactatnt and product side.
Answer:
There is no net ionic equation.