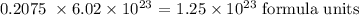Answer:
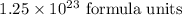
Step-by-step explanation:
Here, we want to calculate the number of formula units in the given molecule
We start by getting the number of moles
To get the number of moles, we have to divide the mass given by the molar mass
The molar mass is the mass per mole
The molar mass of calcium bromide is 200 g/mol
Thus, we have the number of moles as follows:
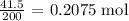
The number of formula units in a mole is:
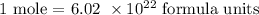
The number of formula units in 0.2075 mole will be: