For us to find out how much charge is being put, we need first to find out how many atoms of sulfur there are. This can be achieved knowing that sulfur has 32.1u of mass, and each u is 1.66*10^-27. So, there will be:
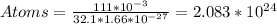
So we'll have a total of 2.083*10^24 atoms. We know that an electron will be added on 1/1012 of these atoms. That is:
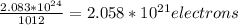
Multiplying this by the charge of each electron:
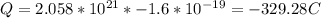
Thus, the new charge will be -329.28C