Given that the density of heptane is
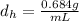
The mass of heptane is
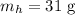
The density of water is
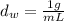
The mass of water is
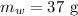
The volume of heptane will be
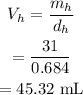
The volume of water will be
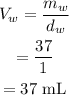
Thus, the volume of heptane is 45.32 mL and the volume of water is 37 mL.
The total volume of liquid in the cylinder will be
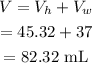
The total volume of liquid in the cylinder will be 82.32 mL.