Answer : There exist an inverse relationship between the wavelength and the energy of the wave.
Explanation :
Ultraviolet radiation is dangerous because it has a high enough energy to damage skin cells.
The energy of a wave is given by the following relation :
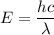
So, it is clear that relation between the wavelength and the energy of the wave is inverse.
Higher the wavelength, least will be the energy of the wave.