Explanation:
The electronic configuration of Calcium and Potassium is written below as
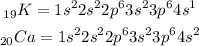
Both calcium and potassium belong to period 4. Since the atomic radius is the distance between the nucleus and the electron moving around the outermost shell of the atom.
Potassium has more shielding than calcium. Hence, having more atomic radius than calcium
Potassium has an atomic number of 19 and calcium has an atomic number of 20. The nuclear charge of 20 0f calcium is shielded by 18 inner electrons while the nuclear charge of 19 potassium atoms is also shielded by 18 inner electrons. Therefore, the outer electrons of calcium are less shielded compared to the potassium atom. The outer electrons in calcium are 2 while Potassium is 1, so, it will be difficult to remove the electrons.
Recall that, both calcium and potassium belong to period 4 in the periodic table. Atomic radius increases down the group when we move from left to right and decreases across the period. As the atomic radius increases, the shielding effect also increases. Since they both have the same period, we can not judge their atomic radius by their group. Since the atomic radius increases from left to right and potassium is on the left to calcium, therefore, the atomic radius of potassium is greater than the atomic radius of calcium