We will determine the moles of base in 16.61mL of the 0.1M base:
If 0.1 mole of the base is in 1000mL then how many moles is is 16.61mL:
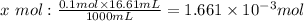
If the molar ratio between the base and the acid is 1:1 then whatsoever moles are in the base would be in the acid. We therefore see that 0.001661mol is in the acid and would be present in the 13.07mL. To determine the molar concentrationof the acid we will determine the moles 1000mL:
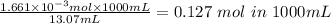
Answer: The concnetration of the acid is 0.127mol/L.