Answer : The enthalpy of fusion of ice is, 334 J
Solution :
Formula used :
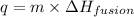
Where,
q = heat absorb = 6.68 KJ = 6680 J
m = mass of ice = 20 g
 = enthalpy of fusion of ice = ?
= enthalpy of fusion of ice = ?
Now put all the given values in this formula, we get the enthalpy of fusion of ice.
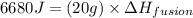
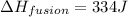
Therefore, the enthalpy of fusion of ice is, 334 J