When a single compound breaks down into two or more compounds or elements in a chemical reaction then it is known as decomposition reaction.
The chemical symbol for sodium carbonate is
 .
.
The decomposition of sodium carbonate is:
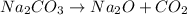
The decomposition of sodium bicarbonate,
 will result in the formation of sodium oxide,
will result in the formation of sodium oxide,
 and carbon dioxide,
and carbon dioxide,
 .
.
Hence, carbon dioxide,
 will produce with sodium oxide,
will produce with sodium oxide,
 on decomposition of
on decomposition of
 .
.