The balanced equation of the combustion of the propane is
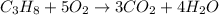
From the above chemical equation, it is clear that 1 mole of propane requires 5 moles of oxygen to undergo complete combustion as the result of this reaction, 3 moles of carbon-di-oxide and 4 moles of water molecules will be produced.
Therefore one-half mole of propane requires 2.5 moles of oxygen to undergo complete combustion. And in the reaction, 1.5 moles of carbon-di-oxide and 2 moles of water molecules are produced.
Therefore the answer is, 'One-half of propane combines with 2.5 moles of oxygen to produce 2 moles of water and 1.5 moles of carbon dioxide'