Answer
Hence, the partial pressure of gas 3 in atm = 0.3 atm
Step-by-step explanation
Given:
Total pressure of the three gases, P(total) = 2.8 atm
Partial pressure of gas 1, P₁ = 480 mmHg
Partial pressure of gas 2, P₂ = 1450 torr
What to find:
The partial pressure of gas 3 in atm.
Step-by-step solution:
According to Dalton's law of partial pressure which states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of the gases in the mixture. i.e
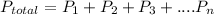
Since the unit of the given partial pressures are not the same with the that of the total pressure, then you need to convert them to atm.
Conversion factor:
760 mmHg = 1 atm
760 torr = 1 atm
Therefore,
The partial pressure of gas 1, P₁ = 480 mmHg = (480 mmHg/760 mmHg) x 1 atm = 0.6 atm
The partial pressure of gas 2, P₂ = 1450 torr = (1450 torr/760 torr) x 1 atm = 1.9 atm
Putting P₁ = 0.6 atm, P₂ = 1.9 atm and P(total) = 2.8 atm into the partial pressure formula above to get the partial pressure of gas 3:
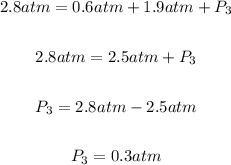
Hence, the partial pressure of gas 3 in atm = 0.3 atm