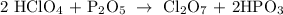First we balance the metals P:phosphorus
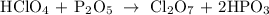
then we balance the non-metals Cl:chlorine
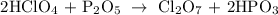
Then we balance the hydrogens and oxygens
If we look at the hydrogens and oxygens they are already balanced because there are 2 hydrogens in the reactants and 2 hydrogens in the products. Similarly, There are 13 oxygens in the reactants and 13 oxygens in the products.
The answer is: