To answer this, we can start at the end and see what we need to calculate it.
We want the number of moles of CO₂ produced. From the chemical equation, we can see that the coefficient of O₂ is 2 and of CO₂ is 1, so wwe need to apply thsi stoichiometry:
CO₂ --- O₂
1 --- 2
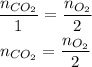
The number of moles of O₂ can be calculated from the mass o O₂ and its molar mass:
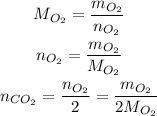
We already have the mass of O₂, but we need to calculate its molar mass:
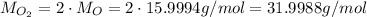
Now, substituting the values, we have:
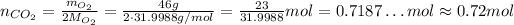
So, the number of moles of CO₂, assuming complete reaction, is approximately 0.72 mol.