Given data
*The given mass is m = 2270 g
*The amount of heat is Q = 5.65 × 10^5 J
*The final temperature of the water is T = 100 C
*The specific heat capacity of water is c = 4.18 J/g C
The formula for the amount of heat required is given as
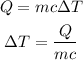
Substitute the values in the above expression as
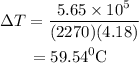
The intial temperature of the water is calculated as
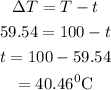
Hence, the initial temperature of the water is 40.46 degree Celsius