Answer:
q₂ = -4.80 10⁻⁴ C = - 0.48 mC, charge is negative
Step-by-step explanation:
Let's use coulomb's law
F =
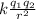
and the sum of forces, remember that charges of the same sign repel and of different sign attract
∑ F = F₁₃ + F₂₃ (1)
Let's start by fixing a reference system located at charge 1 with the positive direction to the right. In the problem it indicates that the net force on charge 3 is F = - 20.0 N, the negative sign indicates that the force is towards the left
let's look for every force, the charge q₁ = 12 10-⁻³ C and q₃ = 15 10⁻³ C
F₁₃ =
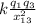
F₁₃ = 9 10⁹ 12.0 15.0 10⁻⁶ / (5-0)²
F₁₃ = 64.8 10 3 N
This force is repulsive, that is, it is directed to the right
F₂₃ = k \frac{q_2 q_3}{x_{23}^2}
F₂₃ = 9 10⁹ q₂ 15.0 10⁻³ / (5-4)²
F₂₃ = 135 10⁶ q₂ N
we substitute in equation 1
-20.0 = 64.8 10³ + 135 10⁶ q₂
q₂ = (-20 - 64.8 10³) / 135 10⁶
q₂ = -4.80 10⁻⁴ C
the sign indicates that the charge is negative