First, we need to find the chemical equation:
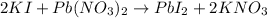
We can identify that the reactants gives a precipitation reaction which mean that there's a product that doesn't dissolve in water.
The net ionic equation shows only the chemical species involved in the reaction.
You can see it by separating the elements of a compound, like this:
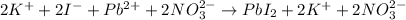
The NO3 looks the same because it doesn't suffer any change during the reaction, while the other reactants do.