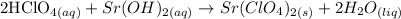We need to write the molecular equation (without ionic species, only molecules) for the neutralization reaction between hydrochloric acid and strontium hydroxide.
Hydrochloric acid (HClO4) and strontium hydroxide (Sr(OH)2) are both strong acid and base, respectively, then we expect that a complete neutralization occurs between these two compounds, yielding to a salt and water.
With that in mind, we can write:
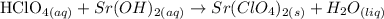
Next, we need to balance the equation: