Since we are given pressure and volume, this tells us that we will be using Boyle's Law.
1) List what you have and what you need.
P₁ = 101.3 kPa
V₁ = 20.0 L
P₂ = 88.4 kPa
V₂ = ?
2) Rearrange your formula and input your values.
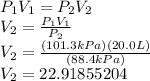
V₂ = 22.9 L
There really isn't much to explain. All you need to do is use the formula and rearrange it so that the variable that you want is isolated and then plug those numbers in the calculator and you have your answer.
To kinda make sure you're doing this right, using the relationship between variables will help you out. You should know that pressure and volume has an inverse relationship so when pressure goes down, you should expect your volume to go up and vice versa. This is the case since the pressure decreased from 101.3 kPa to 88.4 kPa so it makes that the volume will increase.