Answer: Sample A is a pure substance, Sample B is a homogeneous mixture and Sample C is a heterogeneous mixture.
Step-by-step explanation:
Homogeneous mixture : In these mixture, all the component are uniformly distributed throughout the mixture.For example: sugar-water solution.
Heterogeneous mixture : In these mixture, all the component are not uniformly distributed throughout the mixture.For example: sand-water solution.
Pure substance : These are the substance made up only one type of atom or molecule.They have fixed value of boiling and melting points. For example: diamond, pure sugar etc.
Sample A is a clear liquid which evaporates at
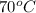 with no change in appearance. Hence, pure substance.
with no change in appearance. Hence, pure substance.
Sample B is a clear blue liquid which boils at
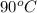 and leaves blue crystals behind with change in appearance. Hence, homogeneous mixture
and leaves blue crystals behind with change in appearance. Hence, homogeneous mixture
Sample C is a opaque, whitish liquid which boils at
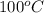 and leaves white crystals behind with dust particles settled in the bottom. Hence, heterogeneous mixture.
and leaves white crystals behind with dust particles settled in the bottom. Hence, heterogeneous mixture.