Hello!
* First Step: to know Avogrado's Law
We know that by the Law of Avogrado, for each mole of substance we have 6.02*10²³ atoms, if:
** Second Step: to know the molar mass of the solute
The molar mass of of magnesium = 24.30 g/mol
*** Third step: make the ratio mass / mol with atoms
1 mol we have 6.02*10²³ atoms
1 mole of Mg we have 24.30 g
Then we have:
24.30 g ------------- 6.02*10²³ atoms
x ----------------------- 6.98*10^24 atoms
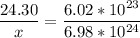
multiply cross
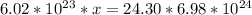
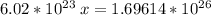
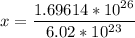
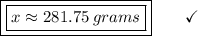
I Hope this helps, greetings ... DexteR! =)