Answer:
20 l of
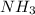
Step-by-step explanation:
the reaction happens under standard conditions (stp) that mean it happens at a temperature of 298 k and 1 atm of pressure
Balanced equation

NO reacts completely means that it is the limit reagent.
This means that the reaction will occur until all the NO is consumed and becomes the products.
we find the moles of NO present in 30 l of compound with the ideal gas equation


By the stoichiometric coefficients, we know that for the reaction to happen we need 6 mol NO for every 4 mol
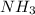
How many moles of
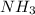 will be necessary to fully react 1.22 mol of NO
will be necessary to fully react 1.22 mol of NO
We apply a simple rule of three

Now with the ideal gas equation we find the liters
