Answer:
b. 4.0 L
Step-by-step explanation:
If we are talking about a gas we may use the Boyle-Mariotte rule which establishes that, at constant temperature, the volume will be inversely proportional to the pressure. i.e. when the pressure increases, the volume decreases and when the pressure decreases, the volume increases. This rule is represented by the equation:
(initial pressure)(initial volume) = (final pressure)(final volume) or
PiVi=PfVf
In the initial state we have a volume of 15.0 L at a pressure of 210 kpa and in the final state we have a pressure of 790 and we need to find out the volume so:
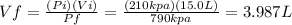
That can be rounded to 4.0 L