Answer: Option (A) is the correct answer.
Step-by-step explanation:
Latent heat of fusion is defined as the heat energy necessary to convert one gram of a solid substance into liquid state.
Molecular formula of water is
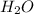 and due to the presence of hydrogen atom there is hydrogen bonding present in water.
and due to the presence of hydrogen atom there is hydrogen bonding present in water.
As boiling point of water is
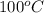 and its heat of vaporization is 40.40.65 kJ/mol. So, it means high heat energy has to be provided in order to break the hydrogen bonding present within the water molecules.
and its heat of vaporization is 40.40.65 kJ/mol. So, it means high heat energy has to be provided in order to break the hydrogen bonding present within the water molecules.
When temperature of water reaches at
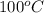 , hydrogen bonding within water molecules tend to break and its molecules gain high kinetic energy.
, hydrogen bonding within water molecules tend to break and its molecules gain high kinetic energy.
This kinetic energy helps the molecules to escape from liquid to gaseous state.
Thus, we can conclude that the statement hydrogen bonds cause water to resist a change in temperature, hydrogen bonds cause water to resist a change in temperature.