Answer:
The length of x is 12.
Step-by-step Step-by-step explanation:
Here's the required formula to find the missing side of triangle :
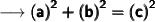
Substituting all the given values in the formula to find the third side of triangle :
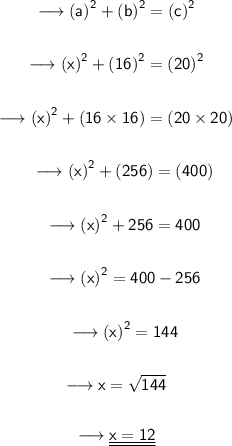
Hence, the length of x is 12.
