Answer:
HF will experience hydrogen bonding
Step-by-step explanation:
Hydrogen bonding (H-bonding) is an intermolecular force having partial ionic-covalent character. H-bonding takes place between a hydrogen atom (attached with an electronegative atom e.g. O, N and F) and an electronegative atom (O,N and F).
Hydrogen bonding generally appears in covalent molecules.
Among all four given molecules, HF and
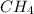 are covalent molecules.
are covalent molecules.
In HF, F is a highly electronegative atom attached to a H atom through a covalent bond. Therefore F atoms gets partial negative charge and H atoms gets partial positive charge.
Hence an interaction arises between H atom and F atom resulting formation of H-bonding.