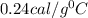Answer:
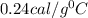
Step-by-step explanation:
The quantity of heat required to raise the temperature of a substance by one degree Celsius is called the specific heat capacity.
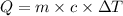
Q = Heat absorbed= 569 calories
m= mass of substance = 155 g
c = specific heat capacity = ?
Initial temperature =
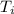 = 25.0°C
= 25.0°C
Final temperature =
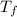 = 40.0°C
= 40.0°C
Change in temperature ,
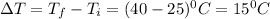
Putting in the values, we get:
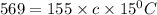
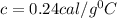
The specific heat of the substance is