You first need to know which formula you are going to use. Since we are given the pressure and volume, this tells us that we will be using Boyle's Law.
You then have to list what you are given and what they are asking for.
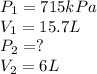
You then need to use the formula to find your missing value.
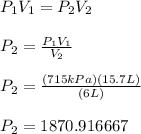
P₂ = 2 x 10³ kPa
Sigdigs(figs) wise, the answer will have one significant digit since the 6L has the least digits.
You can also prove this by knowing the relationship between the values. Pressure and volume has an inverse relationship so it makes sense that the new pressure is a lot higher than the original pressure since our new volume got smaller.
//
Regarding the last question, I have no clue.