Answer: The given compound is formed by silicon and oxygen atoms.
Step-by-step explanation:
We are given a chemical compound having chemical name of silicon dioxide.
This compound is formed by the combination of silicon and oxygen atoms.
Silicon and oxygen, both are non-metals and thus their combination leads to the formation of covalent compound.
A covalent compound is defined as the compound which is formed when two atoms share the electrons while combining.
The chemical formula for silicon dioxide is
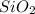
Hence, the given compound is formed by silicon and oxygen atoms.