Answer: Ag +( aq)
Explanation:
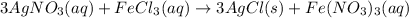
This is an example of double displacement reaction in which exchange of ions take place. Positively charged
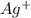 combines with negatively charged
combines with negatively charged
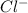 ions to form
ions to form
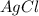 which is insoluble in water, thus forms a precipitate.
which is insoluble in water, thus forms a precipitate.
Positively charged
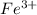 combines with negatively charged
combines with negatively charged
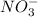 ions to produce
ions to produce
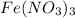 which dissolves in water.
which dissolves in water.