Answer:
3.99 liters of oxygen at STP would be needed to allow complete reaction.
Step-by-step explanation:
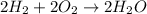
At STP, 1 mol of gas occupies = 22.4 L
Then 1 Liter of volume will be occupied by:
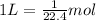
The 8 Liter of volume will be occupied by:
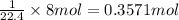
Moles of hydrogen gas = 0.3571 mol
According to reaction, 2 moles of hydrogen gas reacts with 1 mol of oxygen gas.
Then 0.3571 moles of hydrogen gas will react with:
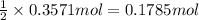 of oxygen
of oxygen
Moles of oxygen required to react completely = 0.1785 moles
At STP, 1 mol of gas occupies = 22.4 L
Then 0.1785 moles of oxygen will occupy:
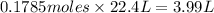
3.99 liters of oxygen at STP would be needed to allow complete reaction.