Answer:
Explanations:
The formula for calculating the mass of a substance is expressed as:
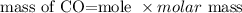
Given the following parameter;
molecules of CO = 1.806 * 10^24 molecules
Convert the molecule to moles
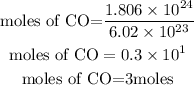
Given that molar mass of CO = 12 + 16 = 28g/mol
Determine the required mass in grams
![undefined]()