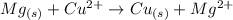The question requires us to write the balanced chemical equation for the reaction between etallic magnesium (Mg) and copper chloride (CuCl2). Also, the question requires the correspondent net ionic reaction.
We'll go through the steps given by the question to solve the problem:
Step 1: The starting chemicals are metallic magnesium, Mg, and copper (II) chloride, CuCl2 (where Cu has oxidation number +2, thus it must bond to two Cl- ions to form copper (II) chloride):
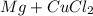
Step 2: The reaction between Mg and CuCl2 produces metallic copper (Cu) and magnesium chloride (MgCl2), following a simple displacement reaction:
product 1: metallic copper -> Cu
product 2: magnesium chloride -> MgCl2
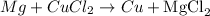
This is a redox reaction (there is a reduction and oxidation ocurring: metallic magnesium, with oxidation number 0, becomes Mg+2 - oxidation - and Cu+2 becomes metallic copper - reduction). Also, this reaction can be classified as a single displacement reaction that happens according to the following scheme: A + BC -> B + AC
Step 3: The reaction written above already presents the same amount of each element on both sides of the equation, thus it is already balanced:
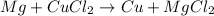
We can write the molecular equation above as the following compelte ionic equation:
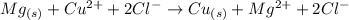
(note that both metallic magnesium and metallic copper are solid metals, thus we can't write them as ions)
We can see from the equation above that the same amount of chlorine ions repeat on both sides of the equation, thus we can cross out these ions (Cl- are spectator ions), and the net ionic equation for this reaction can be written as: