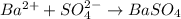Answer:
Spectator ions: Na+ and Br-
Step-by-step explanation:
In a chemical reaction, a spectator ion is a species that does not participate in the reaction and exists in the same form in both the reactants and products side.
The given equation is:
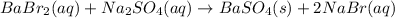
The total ionic equation is
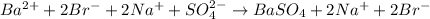
The ions that remain unchanged are: Na+ and Br- ions.
These can be cancelled out on either side to get the net ionic equation: