Answer : The mass of substance in kilograms is 17.5 kg.
Explanation :
Density : It is defined as the mass contained per unit volume.
Formula used for density :
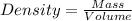
Given :
Volume = 2.50 L
Density of substance = 7.00 g/mL
First we have to convert the volume from liter to milliliters.
Conversion used :
1 L = 1000 mL
As,
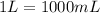
So,
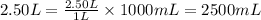
Now we have to calculate the mass of substance.
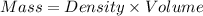
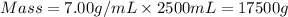
Now we have to convert mass from grams to kilograms.
Conversion used :
1 g = 0.001 kg
As,
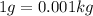
So,
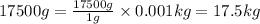
Thus, the mass of substance in kilograms is 17.5 kg.