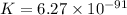Answer : The correct option is, (B)
Solution :
The given balanced redox reaction is,
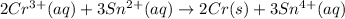
Now we have to calculate the equilibrium constant for the redox reaction.
The relation between the equilibrium constant and cell potential :
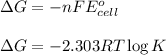
By equation these two equation we get,
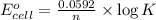 ........(1)
........(1)
where,
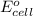 = cell potential = -0.89 v
= cell potential = -0.89 v
n = number of electrons = 6
K = equilibrium constant
Now put all the given values in equation (1), we get
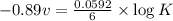
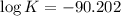
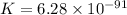
Therefore, the value of the equilibrium constant is,