Answer:
The average kinetic energy of SF₆(g) is 4.98 kj/mol.
Step-by-step explanation:
Given that,
Kinetic energy of UF₆(g)=4.98\ kj/mol
We need to calculate the average kinetic energy of SF₆(g)
Using formula of average kinetic energy
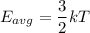
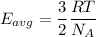
Where, k = Boltzmann constant
 =Avogadro number
=Avogadro number
R =gas constant
Here, average kinetic energy depends only on temperature not on special molar mass
So, The SF₆(g) average kinetic energy is same as UF₆(g
Hence, The average kinetic energy of SF₆(g) is 4.98 kj/mol.