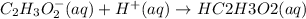Answer:The net ionic equation will be:
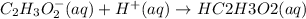
Step-by-step explanation:
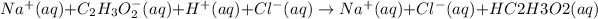
While writing spectator ions got cancelled from equation.Here sodium ions and chloride ion are spectator ions.
Spectator ions are those ions which are present on both the sides of the chemical equation.