Answer: This happens because dry ice sublimates.
Step-by-step explanation:
Dry ice is the solid phase of carbon dioxide and it is a sublimating substance.
Sublimating substance is a substance which undergoes the process of sublimation.
Sublimation is defined as the physical process in which a substance directly changes its state from solid state to gas state.
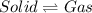
Dry ice directly changes its state from solid (dry ice) to Gas [tex(CO_2)[/tex]. As, carbon dioxide releases, it inflates the balloon.
Hence, dry ice sublimates into carbon dioxide gas and thus inflating the balloon.